Prostate Cancer - Biology, Diagnosis, Pathology, Staging, and Natural History
Updated: Dec 2, 2010
Introduction
Prostate cancer is the most common noncutaneous cancer among males. Lung and bronchial cancer account for 37% of cancer-related death in males; prostate and colon cancers account for another 10% each. The diagnosis and treatment of prostate cancer continue to evolve. With the development of prostate-specific antigen (PSA) screening, prostate cancer is being diagnosed earlier in the disease course. Although prostate cancer can be a slow-growing cancer, thousands of men die of the disease each year. Education is important to help men understand the risk of progression and the various treatment options. This article provides a current overview of the biology, pathology, diagnostic techniques, natural history, and screening of this disorder.
For excellent patient education resources, visit eMedicine's Prostate Health Center and Cancer and Tumors Center. Also, see eMedicine's patient education article Prostate Cancer.
Incidental Findings
In the modern era, most patients present because of abnormalities in a screening PSA level or findings on digital rectal examination (DRE) rather than because of symptoms (see Prostate-Specific Antigen). However, prostate cancer can be an incidental pathologic finding when tissue is removed during transurethral resection to manage obstructive prostatic symptoms (see Prostate Hyperplasia, Benign).
Elevated prostate-specific antigen level
PSA is a single-chain glycoprotein that has chymotrypsinlike properties. PSA slowly hydrolyzes peptide bonds, thereby liquifying semen. The upper limit of normal for PSA is 4 ng/mL. Some advocate age-related cutoffs, such as 2.5 ng/mL for the fifth decade of life, 3.5 ng/mL for the sixth decade of life, and 4.5 ng/mL for the seventh decade of life. Others advocate race-specific reference ranges. Using recent data from screening studies, some have advocated upper limits of normal of 2.5 ng/mL instead of 4 ng/mL.
Prostate-specific antigen velocity
PSA velocity is an important concept. A PSA velocity of lower than 0.75 ng/mL/y has traditionally been used to prompt a prostate biopsy. However, recent data suggest that, among men younger than 50 years, a PSA velocity of 0.6 ng/mL/y may be more appropriate.
Percent of free prostate-specific antigen
The measurement of bound and free PSA is a recent development that can help to differentiate mildly elevated PSA levels due to cancer from elevated levels due to benign prostatic hyperplasia. The lower the ratio of free-to-total PSA, the higher the likelihood of cancer. Free PSA is reported as a percentage. For example, among men with greater than 25% free PSA, only 8% are found to have cancer at prostate biopsy. In contrast, more than half of men with less than 10% free PSA are found to have cancer at biopsy. While cutoffs may be used, the percentage of free PSA is usually used as an additional factor in making an informed recommendation for or against biopsy. Generally, these percentages are useful in patients who have a PSA level in the range of 4-10 ng/mL.
This information is most useful in men with very large glands or in men in whom one biopsy result has already been negative. In healthy men with a PSA level of 4-10 ng/mL, many recommend biopsy without the additional free-PSA test or consider a trial of antibiotic therapy for 4-6 weeks before repeating the PSA test. If antibiotic therapy quickly lowers the PSA level to within the reference range, the cause of the prior elevation is less likely to be prostate cancer, and the PSA test should be repeated within a few months.
Abnormal digital rectal examination findings
Various factors are considered when a DRE is performed. A nodule is important, but findings such as asymmetry, difference in texture, and bogginess are important clues to the patient's condition and should be considered in conjunction with the PSA level. Change in texture over time can offer important clues about the need for intervention. Cysts or stones cannot be accurately differentiated from cancer based on DRE findings alone; therefore, maintain a high index of suspicion if the DRE results are abnormal. In addition, if cancer is detected, the DRE findings form the basis of clinical staging of the primary tumor (ie, tumor [T] stage in the tumor node metastases [TNM] staging system). In current practice, the DRE results are normal but the PSA readings are abnormal in most patients diagnosed with prostate cancer.
Local Symptoms
In the pre-PSA era, patients with prostate cancer commonly presented with local symptoms. Urinary retention developed in 20-25% of these patients, back or leg pain developed in 20-40%, and hematuria developed in 10-15%. Currently, with PSA screening, patients report urinary frequency (38%), decreased urine stream (23%), urinary urgency (10%), and hematuria (1.4%). However, none of these symptoms is unique to prostate cancer and each could arise from various other ailments. Forty-seven percent of patients are asymptomatic.
Metastatic Symptoms
Metastatic symptoms include weight loss and loss of appetite; bone pain, with or without pathologic fracture (because prostate cancer, when metastatic, has a strong predilection for bone); and lower extremity pain and edema due to obstruction of venous and lymphatic tributaries by nodal metastasis. Uremic symptoms can occur from ureteral obstruction caused by local prostate growth or retroperitoneal adenopathy secondary to nodal metastasis.
Frequency
With the advent of PSA screening, a greater number of men require education about prostate cancer and how it is diagnosed, staged, and treated so they can select the most appropriate treatment.
According to figures from the American Cancer Society, 186,330 new cases will be diagnosed in 2008 and 26,000 men will die from prostate cancer (see Image 1). Prostate cancer is rarely diagnosed in men younger than 40 years, and it is uncommon in men younger than 50 years.
Prevalence rates of prostate cancer remain significantly higher in African American men than in white men, while the prevalence in Hispanic men is similar to that of white men. Hispanic men and African American men present with more advanced disease, most likely related to external (eg, income, education, insurance status) and cultural factors. In addition, African American men generally have higher levels of testosterone, which may contribute to the higher incidence of carcinoma.
Between 1989 and 1992, incidence rates of prostate cancer increased dramatically, probably because of earlier diagnoses in asymptomatic men as a result of the increased use of serum PSA testing. In fact, the incidence of organ-confined disease at diagnosis has increased because both PSA testing and standard DRE are performed.
Prostate cancer incidence rates are continuing to decline; rates in white men peaked in 1992, and they peaked in African American men in 1993.
During 1992-1996, mortality rates associated with prostate cancer declined significantly, approximately 2.5% per year (see Image 1). Although mortality rates are continuing to decline among white and African American men, mortality rates in African American men remain twice as high as in white men, based on 2008 American Cancer Society projections.
Prostate cancer is also found during autopsies performed following other causes of death. The rate of this latent or autopsy cancer is much greater than that of clinical cancer. In fact, it may be as high as 80% by age 80 years.
The prevalence of clinical cancer varies by region, and these differences may be due to some of the genetic, hormonal, and dietary factors discussed in Etiology. High rates are reported in northern Europe and North America, intermediate rates are reported in southern Europe and Central and South America, and low rates are reported in Eastern Europe and Asia.
Interestingly, the prevalence of the latent or autopsy form of the disease is similar worldwide. Together with migration studies, this suggests that environmental factors, such as diet, may play a significant promoting role in the development of a clinical cancer secondary to a latent precursor.
Etiology
Genetics
Gene alterations on chromosome 1, 17, and the X chromosome have been found in some patients with a family history of prostate cancer. The hereditary prostate cancer 1 (HPC1) gene and the predisposing for cancer of the prostate (PCAP) gene are on chromosome 1, while the human prostate cancer gene is on the X chromosome. In addition, genetic studies suggest that a strong familial predisposition may be responsible for as many as 5-10% of prostate cancer cases. Recently, several reports have suggested a shared familial risk (inherited or environmental) for prostate and breast cancer. Men with a family history of prostate cancer have a higher risk of developing prostate cancer and are also likely to present 6-7 years earlier.
Race
African American men have a higher prevalence and more aggressive prostate cancer than white men, who, in turn, have a higher prevalence than men of Asian origin. Studies have found that young African American men have testosterone levels that are 15% higher than in young white men. Furthermore, evidence indicates that 5-alpha reductase may be more active in African Americans than in whites, implying that hormonal differences may play a role. The independent contribution of race alone is difficult to qualify when the effects of health care access, income, education, and insurance status are also considered.
Diet
A high-fat diet may lead to increased risks, while a diet rich in soy may be protective. These observations have been proposed as reasons for the low prevalence of this cancer in Asia. Rates of prostate cancer are much greater in Japanese American men than in native Japanese men, supporting the association of a high-fat diet with cancer. Cell culture studies have shown that omega-6 fatty acids are positive stimulants of prostate cancer cell growth, while omega-3 fatty acids are negative stimuli. These fats may exert their effects by alterations of sex hormones or growth factors or through effects on 5-alpha reductase.
Soy seems to decrease the growth of prostate cancer cells in mouse models; however, apart from epidemiologic factors, no direct evidence supports a beneficial effect in humans. Vitamin E may have some protective effects because it is an antioxidant. Decreased levels of vitamin A may be a risk factor because this can promote cell differentiation and stimulate the immune system. Vitamin D deficiency was suggested as a risk factor, and studies show an inverse relationship between ultraviolet exposure and mortality rates for prostate cancer. However, a specific correlation between 1,25-dihydroxyvitamin D levels and palpable disease, well-differentiated tumors, or mortality is inconclusive.
Selenium may have a protective effect based on epidemiologic studies and is also believed to extend its effect via its antioxidant properties. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) is an ongoing intergroup, phase 3, randomized, controlled trial designed to test the efficacy of selenium and vitamin E alone and in combination in the prevention of prostate cancer.
For more information, see Prostate Cancer: Nutrition.
Hormones
Hormonal causes have also been postulated. Androgen ablation causes a regression of prostate cancer. In addition, as indirect evidence of hormonal causes, eunuchs do not develop adenocarcinoma of the prostate.
Hsing and Comstock performed a large study comparing patients with prostate cancer with controls and found no difference in levels of testosterone, dehydrotestosterone, prolactin, follicle-stimulating hormone, or estrone.1
A 2010 case-cohort study suggested that higher serum estrone levels were linked to an increased risk of incident prostate cancer. However, this link between the sex hormone and prostate cancer needs further investigation.2
The Prostate Cancer Prevention Trial studied the prevalence of prostate cancer between a control group and a group given a 5-alpha-reductase inhibitor (finasteride). While the 5-alpha reductase inhibitor appeared to decrease the prevalence of tumors, those that did arise appeared histologically more aggressive. Only long-term follow-up of these patients will determine whether this more aggressive histology accurately reflects the underlying biology of these tumors or whether it is an artifact of the treatment.
The American Society of Clinical Oncology (ASCO) Health Services Committee (HSC), ASCO Cancer Prevention Committee, and the American Urological Association Practice Guidelines Committee jointly convened a Panel of experts who used the results from a systematic review of the literature to develop evidence-based recommendations on the use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention.The Expert Panel concluded that asymptomatic men with a PSA level of less than 3 ng/mL who are regularly screened with PSA or are anticipating undergoing annual PSA screening for early detection of prostate cancer may benefit from a discussion of both the benefits of 5-alpha-reductase inhibitors for 7 years for the prevention of prostate cancer and the potential risks (including the possibility of high-grade prostate cancer).
Men who are taking 5-alpha-reductase inhibitors for benign conditions, such as lower urinary tract (obstructive) symptoms (LUTS), may benefit from a similar discussion; these patients should understand that the improvement of LUTS relief should be weighed with the potential risks of high-grade prostate cancer from 5-alpha-reductase inhibitors (although most of the Panel members judged the risk of high-grade prostate cancer to be unlikely). A reduction of approximately 50% in PSA level by 12 months is expected in men taking a 5-alpha-reductase inhibitor; however, because these changes in PSA may vary among men, and within individual men over time, the Panel has no recommendations for a specific cut point to trigger a biopsy for men taking a 5-alpha-reductase inhibitor. No specific cut point or change in PSA level has been prospectively validated in men taking a 5-alpha-reductase inhibitor.3
Pathophysiology and Natural History
Pathophysiology
Prostate cancer develops when the rates of cell division and cell death are no longer equal, leading to uncontrolled tumor growth. Following the initial transformation event, further mutations of a multitude of genes, including the genes for p53 and retinoblastoma, can lead to tumor progression and metastasis. Most (95%) prostate cancers are adenocarcinomas.
Approximately 4% of cases of prostate cancer have transitional cell morphology and are thought to arise from the urothelial lining of the prostatic urethra. Few cases have neuroendocrine morphology. When present, they are believed to arise from the neuroendocrine stem cells normally present in the prostate or from aberrant differentiation programs during cell transformation.
Of prostate cancer cases, 70% arise in the peripheral zone, 15-20% arise in the central zone, and 10-15% arise in the transitional zone. Most prostate cancers are multifocal, with synchronous involvement of multiple zones of the prostate, which may be due to clonal and nonclonal tumors.
Natural history
The natural history is still relatively unknown, and many aspects of progression are poorly understood. Symptoms or abnormal DRE findings in the pre-PSA era brought only 40-50% of patients with prostate cancer to medical attention, and these patients usually had locally advanced disease. The advent of PSA testing has helped to identify patients with less-advanced, organ-confined disease.
In fact, the pendulum has shifted to the point that certain members of the urologic community feel that active surveillance, also known as expectant management, may have a role. Twenty-year outcome data from Connecticut confirm that mortality rates due to tumors with a Gleason score of 2-4 was less than 7%.4 Urologists at Johns Hopkins University advocate active surveillance in patients with a PSA density of less than 0.1 ng/mL, with no adverse pathologic findings on needle biopsy, and with tumors with a Gleason score of 6 that are smaller than 3 mm.
Evidence suggests that most prostate cancers are multifocal and heterogeneous. Cancers can start in the transitional zone or, more commonly, the peripheral zone. When these cancers are locally invasive, the transitional-zone tumors spread to the bladder neck, while the peripheral-zone tumors extend into the ejaculatory ducts and seminal vesicles. Penetration through the prostatic capsule and along the perineural or vascular spaces occurs relatively late.
The mechanism for distant metastasis is poorly understood. The cancer spreads to bone early, occasionally without significant lymphadenopathy. Currently, 2 predominant theories have been proposed for spread—the mechanical theory and the seed-and-soil theory.
- The mechanical theory involves direct spread through the lymphatics and venous spaces into the lower lumbar spine.
- Advocates of the seed-and-soil theory believe that tissue factors that allow for preferential growth in certain tissues, such as the bone, must be present. Lung, liver, and adrenal metastases have also been documented. Specific tissue growth factors and extracellular matrices are possible examples.
The doubling time in early-stage disease is as slow as 2-4 years, but this changes as the tumor grows and becomes more aggressive. Larger tumors usually have a higher Gleason grade and a faster doubling time.
Natural history by stage
- T1a - Progression over 10 years (uncommon)
- T1b - Tumor-related death rate of 10% in 10 years
- T2 - Ten-year metastasis-free survival rate of 81% with grade 1, 58% with grade 2, and 26% with grade 3
- T3 - Lymph node metastasis at presentation in 50% and approximately 25% rate of 10-year disease-free survival
The natural history of clinically localized disease varies, with lower-grade tumors having a more indolent course, while some high-grade lesions progress to metastatic disease with relative rapidity. Several studies have examined the cancer-specific and quality-of-life outcomes associated with a watchful-waiting approach to localized disease.
- Albertsen et al monitored patients who received no initial treatment for prostate cancer.4 As disease progression occurred, many received antiandrogens. Men with poorly differentiated tumors lost 6-8 years of life, while those with moderately differentiated tumors lost 4-5 years. Of all men monitored for 10 years, 40% died of causes other than prostate cancer. This study was performed prior to PSA screening.
- Graversen et al compared watchful waiting with radical prostatectomy.5 They found no overall difference in survival, but they did find that a high Gleason score was associated with poor survival in both groups.
- Chodak et al confirmed this finding by analyzing 6 studies and finding a 34% survival rate associated with grade 3 tumors versus an 87% disease-specific survival rate associated with grade 1 and 2 tumors.6 The metastasis-free survival rate also significantly dropped as the grade progressed from 1 to 3.
- Johansson et al (2004) reported their recent update on a population-based cohort study with a mean observation period of 21 years.7 In this study, 223 patients with early-stage, initially untreated prostatic cancer were observed. Symptomatic patients with tumor progression received hormonal treatment (orchiectomy or estrogens). Thirty-nine (17%) developed metastatic disease, with most cancers having an indolent course during the first 10-15 years. However, further follow-up at 15-20 years revealed a substantial decrease in cumulative progression-free survival (from 45% to 36%), survival without metastases (from 76.9% to 51.2%), and prostate cancer–specific survival (from 78.7% to 54.4%). Prostate cancer mortality increased from 15 deaths per 1000 person-years during the first 15 years to 44 deaths per 1000 person-years beyond 15 years of follow-up.
Taken together, these data suggest that, although most prostate cancers diagnosed at an early stage have an indolent course, local tumor progression and aggressive metastatic disease may develop in the long term. In addition, these findings would support early radical treatment, notably among patients with an estimated life expectancy exceeding 15 years.
Screening
DRE and PSA evaluation are the 2 components necessary for a modern screening program. Transrectal ultrasonography (TRUS) has been associated with a high false-positive rate, making it unsuitable as a screening tool, although it is very useful for directing prostatic biopsies.
The indications for screening are controversial. The American Cancer Society recommends that both PSA evaluation and DRE should be offered annually, beginning at age 50 years, to men who have at least a 10-year life expectancy and to high-risk younger men. Information should be provided to patients regarding potential risks and benefits of intervention.
Despite the apparent survival advantage of early diagnosis conferred by PSA screening, a recent U.S. Preventive Services Task Force statement recommends against screening for prostate cancer in men aged 75 years or older. The statement also concludes that, currently, the balance of benefits versus drawbacks of prostate cancer screening in men younger than age 75 years cannot be assessed because of insufficient evidence.8
A 2010 study found that in the 75-80 year age group, African American men with an initial PSA measurement of less than 6.0 ng/mL and Caucasian men with an initial PSA measurement of less than 3.0 ng/mL are unlikely to develop high-risk prostate cancer or die from prostate cancer. Based on these results, the authors conclude that discontinuation of PSA screening in this group may be safe.9
Advocates of screening believe that early detection is crucial to finding organ-confined disease and to reducing the likelihood of mortality. When symptoms develop or when DRE results become positive, most cases have already advanced beyond organ-confined disease. Those who do not advocate screening worry that screening will detect cancers that are not biologically significant (ie, in patients who will die with prostate cancer rather than from it). Currently, age-specific PSA cutoffs are used to guide screening. The trend is toward lowering the threshold level to 2.5 ng/mL, but this has not yet been widely accepted.
Men who choose to undergo screening should begin at age 50 years. Men in high-risk groups, such as African Americans and those with a strong familial predisposition (2 or more affected first-degree relatives), should begin screening at a younger age (40-45 y). These men are less likely to have the latent form of the disease and benefit from treatment. More data on the precise age to start prostate cancer screening are needed for men at high risk.
Recent data from Canadian and Austrian studies suggest that mortality rates are lower as a result of PSA screening. Canadian data have shown that, from 1989-1996, the mortality rate was lower in the PSA-screened cohort than in the control group. Recent studies from Tyrol, Austria, also show a beneficial result for screening in reducing disease-specific mortality. These beneficial effects are likely due to the fact that treatment rather than observation may enhance disease-specific survival. This was recently shown in a 2002 Scandinavian study, which reported that radical prostatectomy was associated with significantly reduced disease-specific mortality compared with watchful waiting. No difference in overall survival was noted.
Currently, US data have shown a mortality rate decrease of 1% per year since 1990, which coincides with the advent of PSA screening. Other theories have been proposed to account for the decrease, and these include changing treatment practices and artifacts in mortality rates secondary to the changing incidence.
Abnormal rectal examination findings
Findings from the DRE are crucial. An irregular firm prostate or nodule is typical, but many cancers are found in prostates that feel normal. Pay careful attention to the prostate consistency, along with the seminal vesicles and adjacent organs, to detect spread of the disease to these structures.
- Overdistended bladder due to outlet obstruction
- Neurologic findings secondary to cord compression: Other subtle findings, such as paresthesias or wasting, are uncommon.
- Lower extremity lymphedema
- Supraclavicular adenopathy
- Lower extremity deep venous thrombosis
- Cancer cachexia
Transrectal ultrasonography
TRUS is used to examine the prostate for hypoechoic areas, which are commonly associated with cancers but are not specific enough for diagnostic purposes. At least 6 or, more recently, 10 or more systematic biopsy specimens of peripheral and, occasionally, transitional zones are taken under ultrasonographic guidance. Samples should include most areas of the gland, irrespective of ultrasonographic abnormalities.
Transrectal sonogram of the prostate showing a hypoechoic lesion in the peripheral zone of the gland that is suggestive of cancer.
Differential diagnoses
- Benign prostatic hypertrophy
- Calculi
- Prostatic cysts
- Prostatic tuberculosis
- Prostatitis
Staging
The 2002 TNM staging system is used to stage prostate cancer, as follows:
- T - Primary tumor
- TX - Primary tumor cannot be assessed
- T0 - No evidence of primary tumor
- T1 - Clinically inapparent tumor not palpable or visible by imaging
- T1a - Tumor incidental histologic finding in less than or equal to 5% of tissue resected
- T1b - Tumor incidental histologic finding in greater than 5% of tissue resected
- T1c - Tumor identified by needle biopsy (because of elevated PSA level); tumors found in 1 or both lobes by needle biopsy but not palpable or reliably visible by imaging
- T2 - Tumor confined within prostate
- T2a - Tumor involving less than half a lobe
- T2b - Tumor involving less than or equal to 1 lobe
- T2c - Tumor involving both lobes
- T3 - Tumor extending through the prostatic capsule; no invasion into the prostatic apex or into, but not beyond, the prostatic capsule
- T3a - Extracapsular extension (unilateral or bilateral)
- T3b - Tumor invading seminal vesicle(s)
- T4 - Tumor fixed or invading adjacent structures other than seminal vesicles (eg, bladder neck, external sphincter, rectum, levator muscles, pelvic wall)
- NX - Regional lymph nodes (cannot be assessed)
- N0 - No regional lymph node metastasis
- N1 - Metastasis in regional lymph node or nodes
Regional lymph nodes are assessed via surgical removal or biopsy of the pelvic lymph nodes, including the obturator chain. The surgical boundaries include the bifurcation of the common iliac, the obturator nerve, and the node of Cloquet.
Distant metastasis
- PM1c - More than 1 site of metastasis present
- MX - Distant metastasis cannot be assessed
- M0 - No distant metastasis
- M1 - Distant metastasis
- M1a - Nonregional lymph node(s)
- M1b - Bone(s)
- M1c - Other site(s)
Workup and Histologic Findings
Workup
Determine the PSA level. Age-related PSA levels can be assessed, as can clinical evidence of prostatitis. If the physician believes that an elevated PSA level may be due to infection, 4-6 weeks of antibiotics are provided, and then the PSA level is rechecked.
Perform a DRE. This is examiner-dependent, and serial examinations, over time, are best. Regard nodules or changes in the texture or the level of asymmetry with a high index of suspicion. Physical examination findings alone cannot reliably differentiate a cyst or calculus from cancer foci; therefore, a biopsy is warranted in these circumstances.
Perform a biopsy to aid in diagnosis and determine the Gleason score. Antibiotics are administered, and an enema is often provided before the procedure, followed by a short course of antibiotics after the biopsy. Coagulation tests are not routinely performed, but patients are instructed to stop aspirin and nonsteroidal anti-inflammatory drugs 10 days prior to the biopsy. Many physicians use lidocaine prior to the biopsy, while others do not. The number of biopsies that should be performed is debated. Sextant versus 12- versus 18-core biopsy protocols are published in the literature. The 12- or 18-core protocols yield more specimens from the lateral regions and usually sample the transition zone. Several studies have demonstrated an increase in the cancer detection rate, while others have not.
In patients with a persistently elevated PSA level in the face of negative biopsy results, the literature supports repeating the biopsy once or twice. Of cancer cases, 31% were detected on repeat biopsy and 39% were detected if the PSA value was greater than 20 ng/mL. If all the biopsy results are negative, a repeat round of biopsies has been suggested when the PSA increases by 25% from the level at which the last biopsies were performed.
Further workup depends on the clinical staging. A higher clinical stage of cancer determined by DRE findings, PSA level, and Gleason score (as determined by biopsy) correlates with an increased risk of extraprostatic spread, and these tests are considered key factors in determining the staging workup and predicting patient prognosis.
The Partin tables are the best nomogram for predicting prostate cancer spread and prognosis. In addition, a series of nomograms has been issued from the Memorial Sloan-Kettering Cancer Center; these nomograms are used to predict biochemical-free survival after surgery and radiation. The most commonly used is the Kattan nomogram.
Men with PSA levels less than 10 ng/mL and low- or moderate-grade histology (Gleason score <7)>
MRI is superior to bone scan in evaluating bone metastasis but is impractical for routine total-body surveys. Instead, it is used to determine the etiology of questionable lesions found on bone scans. MRI is promising for local staging but is not readily accessible, and no published guidelines are available.
Anterior and posterior bone scans of a patient with prostate cancer, with metastasis to the 12th rib and thoracic spine represented by the increased uptake of isotope.
Neither CT scanning nor MRI can be used to determine if lymph nodes are reactive or contain malignant deposits unless the nodes are significantly enlarged and a percutaneous biopsy can be performed.
There is increasing interest in using metabolic activity to detect cancer foci. Positron emission tomography (PET) uses glucose analogue 18 F-fluorodeoxyglucose (18 F-FDG) to detect cancer, but studies thus far have been disappointing for prostate cancer detection.
C-choline PET scans fused with CT imaging show more promise but are not yet the standard of care. Likewise, there is renewed interest in ProstaScint scans fused with MRI or CT images. This modality involves a murine monoclonal antibody that reacts with prostate-specific membrane antigen to identify cancer both in the prostate and in metastatic deposits.
Finally, conventional endorectal MRI is helpful for localizing cancer within the prostate and seminal vesicles and for local staging. Dynamic contrast-enhanced MRI and MR spectroscopic imaging are also complementary in local staging, but their use is currently limited to a research setting.
Preoperative workup includes the following:
- Chest radiography
- CBC count
- CHEM-7
- Prothrombin time and activated partial thromboplastin time
- Electrocardiography
Histologic findings
The most commonly used system of classifying histologic characteristics of prostate cancer is the Gleason score, which is determined using the glandular architecture within the tumor.
The predominant pattern and the second most common pattern are given grades from 1-5. The sum of these 2 grades is referred to as the Gleason score. Scoring based on the 2 most common patterns is an attempt to factor in the considerable heterogeneity within cases of prostate cancer. In addition, this scoring method was found to be superior for predicting disease outcomes compared with using the individual grades alone.
Histologic scoring system showing the 2 most common patterns seen on the biopsy specimen, termed the Gleason score.
Grades are based on the extent to which the epithelium assumes a normal glandular structure. A grade of 1 indicates a near-normal pattern, and grade 5 indicates the absence of any glandular pattern (less malignant to more malignant). This scheme of grading histological features greatly depends on the skill and experience of the pathologist and is subject to some degree of individual variation.
- A score of 2-4 is considered low grade or well differentiated.
- A score of 5-7 is considered moderate grade or moderately differentiated.
- A score of 8-10 is considered high grade or poorly differentiated.
Although the change in glandular architecture represented by the Gleason score is currently the most widely used and correlative histological parameter, it is not the only histological change that can be observed in prostate cancers. Indeed, notable changes in cell and nuclear morphology, neuroendocrine differentiation, and vascularity can be observed and may have great prognostic significance.
Perineural invasion is an indicator of invasiveness and is considered in terms of which side should possibly undergo a nerve-sparing procedure and whether a patient might benefit more from high- or low-risk brachytherapy.
Prostatic intraepithelial neoplasia (PIN) represents the putative precancerous end of the morphologic continuum of cellular proliferations within prostatic ducts, ductules, and acini.
Two grades of PIN are identified. Low-grade PIN is mild dysplasia. High-grade PIN encompasses moderate and severe dysplasia. High-grade PIN is considered by most to be a precursor of invasive carcinoma. Men with high-grade PIN alone can be started on finasteride and monitored closely.
The continuum that culminates in high-grade PIN and early invasive cancer is characterized by basal cell layer or basement membrane disruption, progressive loss of secretory differentiation markers, increasing nuclear and nucleolar abnormalities, increasing proliferative potential, and increasing variation in DNA content (aneuploidy).
Clinical studies suggest that PIN predates a carcinoma by 10 or more years. The clinical importance of recognizing PIN is based on its strong association with carcinoma. Studies claim that men with high-grade PIN in a prostate biopsy specimen have a 35-50% chance of being diagnosed with prostate cancer after a subsequent biopsy.10 Atypical small acinar proliferation (ASAP) has also been associated with higher cancer detection rates. The identification of PIN in prostate biopsy specimens warrants further searching for concurrent invasive carcinoma. In most men, this means repeat biopsies if the PSA level changes significantly. The same may also be true for ASAP findings after biopsy.
Relevant Anatomy
See Image 2. The prostate lies below the bladder and encompasses the prostatic urethra. It is surrounded by a capsule and is separated from the rectum by a layer of fascia termed the Denonvilliers aponeurosis.
The blood supply to the base of the bladder and prostate is from the inferior vesical, which is derived from the internal iliac. The capsular branches of the inferior vesical artery help identify the pelvic plexus arising from the S2-S4 and T10-T12 nerve roots.
The neurovascular bundle lies on either side of the prostate on the rectum. It is derived from the pelvic plexus and is important for erectile function.
Future and Controversies
Whether one of the several different modalities used for treating localized prostate cancer offers survival benefits over another remains controversial. The choice of definitive therapy has been suggested to make a significant difference in long-term survival in less than 10% of patients. This means that most patients are either cured by any definitive therapy or present with incurable disease that cannot be detected, and, ultimately, any treatment modality fails to be curative.
The combination of docetaxel, estramustine, and bevacizumab for the treatment of castrate-resistant prostate cancer (CRPC) encourages antitumor activity, but toxicity complications warrant further evaluation of the efficacy of these drugs. Phase 3 trials are ongoing.11
A 2008 research summary by the Agency for Healthcare Research and Quality (AHRQ) concluded that no single therapy can be considered the preferred treatment for presumed organ-confined prostate cancer. The AHRQ based this conclusion partly on the lack of data regarding efficacy and partly on the concept that differences in adverse effects, convenience, and costs among the available therapies may be important factors in the choice of treatment in an individual patient. The AHRQ noted that, although all treatment options carry adverse effects, patient satisfaction with therapy is high.12
Molecular prognostic markers
Over the past few years, several molecular markers have been shown to aid in the prognostication of patients undergoing treatment for localized and metastatic prostate cancers. Assessment of the molecular alterations or gene products of TP53, RB, BCL2, cathepsin-D, CDH1, and PTEN, among many others, have been reported. Prospective trials are needed to assess these markers more thoroughly before their implementation in current management is recommended.
Reverse transcriptase-polymerase chain reaction
Reverse transcriptase-polymerase chain reaction (RTPCR) testing may be able to find very small amounts of PSA nucleic acid in the blood stream, prostatic fossa, or bone marrow. In the future, this may be helpful in determining which patients have residual tumor following surgery (RTPCR-positive prostate fossa) or a higher rate of tumor recurrence (RTPCR-positive lymph nodes at surgery or persistently positive bone marrow samples months after treatment).
Multimedia
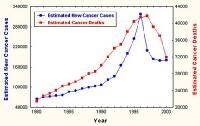 | Media file 1: Estimated incidence and mortality from prostate cancer. Courtesy of the American Cancer Society. |
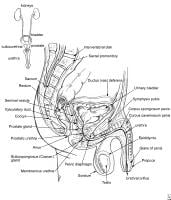 | Media file 2: Relevant anatomy of the male pelvis and genitourinary tract. |
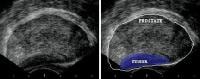 | Media file 3: Transrectal sonogram of the prostate showing a hypoechoic lesion in the peripheral zone of the gland that is suggestive of cancer. |
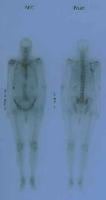 | Media file 4: Anterior and posterior bone scans of a patient with prostate cancer, with metastasis to the 12th rib and thoracic spine represented by the increased uptake of isotope. |
 | Media file 5: Histologic scoring system showing the 2 most common patterns seen on the biopsy specimen, termed the Gleason score.
|

No comments:
Post a Comment